Categories
- Blog (7)
Retatrutide (RETA) is a glucose dependent insulin-like peptide (GIP), glucagon like peptide-1 (GLP-1), and glucagon (GCG) multiple receptor agonist. Previous studies have shown that once weekly injection of Retatrutide can significantly improve blood glucose control and lipid metabolism in patients with type 2 diabetes (T2D), and also has a strong weight loss effect!
On June 26, the 83rd American diabetes Association (ADA) academic conference released the latest clinical trial data of Relatrutide in the treatment of obesity and nonalcoholic fatty liver disease (NAFLD). On the same day, the New England Journal of Medicine (NEJM) published online the results of a phase 2 clinical trial of Retatrutide in the treatment of obesity.
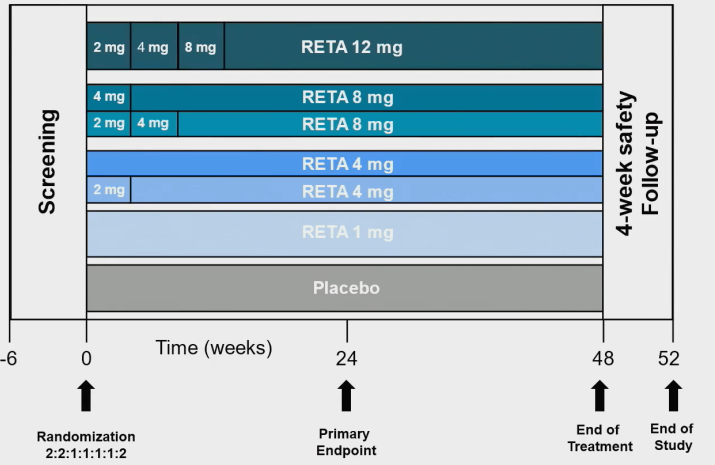
This randomized, double-blind phase 2 clinical trial evaluated the weight loss effect and safety of placebo and different doses of Retatrutide after 48 weeks of treatment.
In the study, adults with BMI ≥ 30 kg/m2 or BMI 27-30 kg/m2 and at least one weight related disease (except for diabetes) were randomly allocated and treated with Rotarutide injection at a ratio of 2:1:1:1:2:2. The doses were 1 mg, 4 mg (initial dose 2 mg), 4 mg (initial dose 4 mg), 8 mg (initial dose 2 mg), 8 mg (initial dose 4 mg), 12 mg (initial dose 2 mg), respectively.
The main endpoint of clinical trials is the percentage change in body weight from baseline to 24 weeks. Secondary endpoints include the percentage of weight change from baseline to 48 weeks, the proportion of weight loss ≥ 5%, ≥ 10%, ≥ 15%, and the safety of treatment.
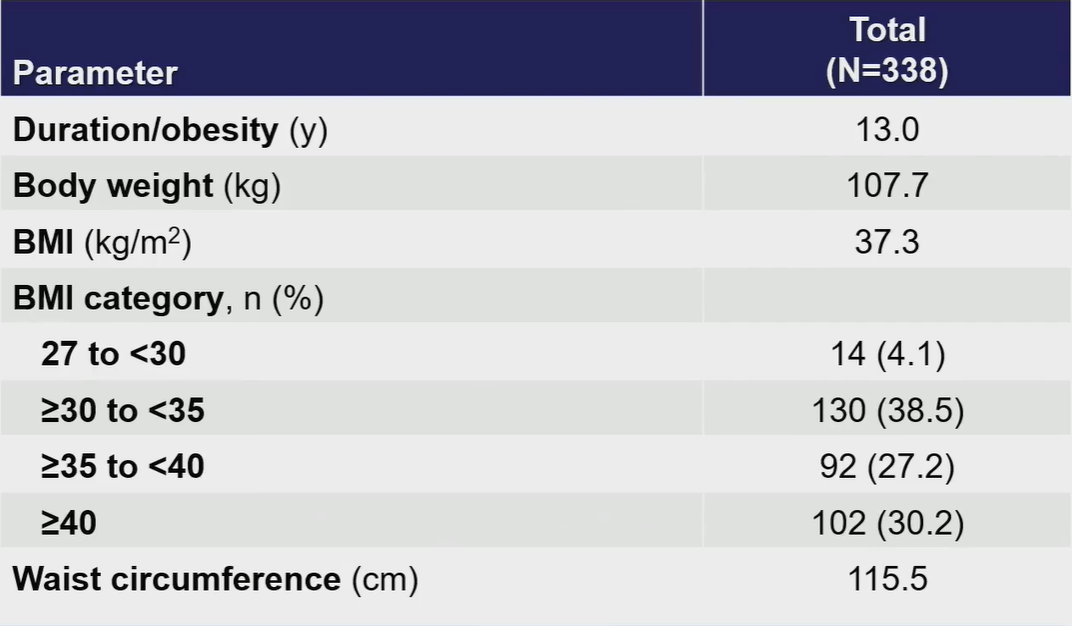
The average age of the 338 enrolled patients was 48.2 years, with 51.8% being males. There was no significant difference in baseline characteristics among different treatment groups.
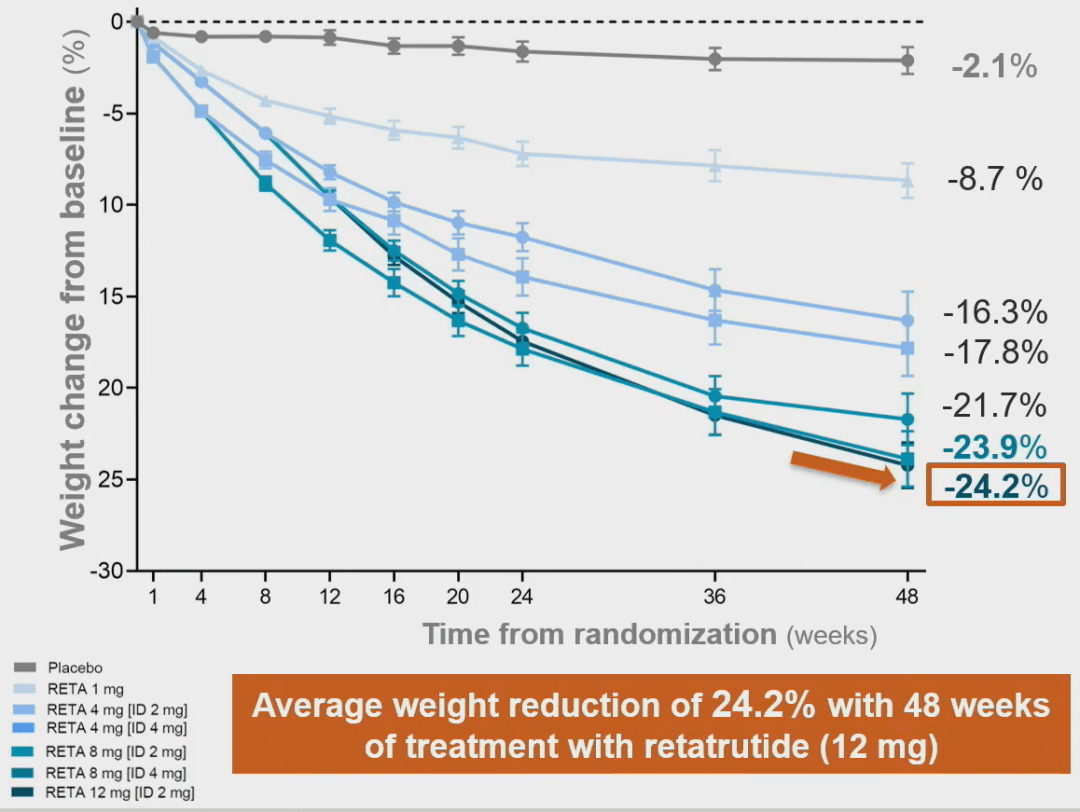
Compared to placebo, all doses of Retatrutide treatment showed clinically significant weight loss and had better weight loss effects on women.
The weekly injection of Retatrutide (12 mg) resulted in an average weight loss of 24.2% after 48 weeks, which is the best drug weight loss effect achieved so far.
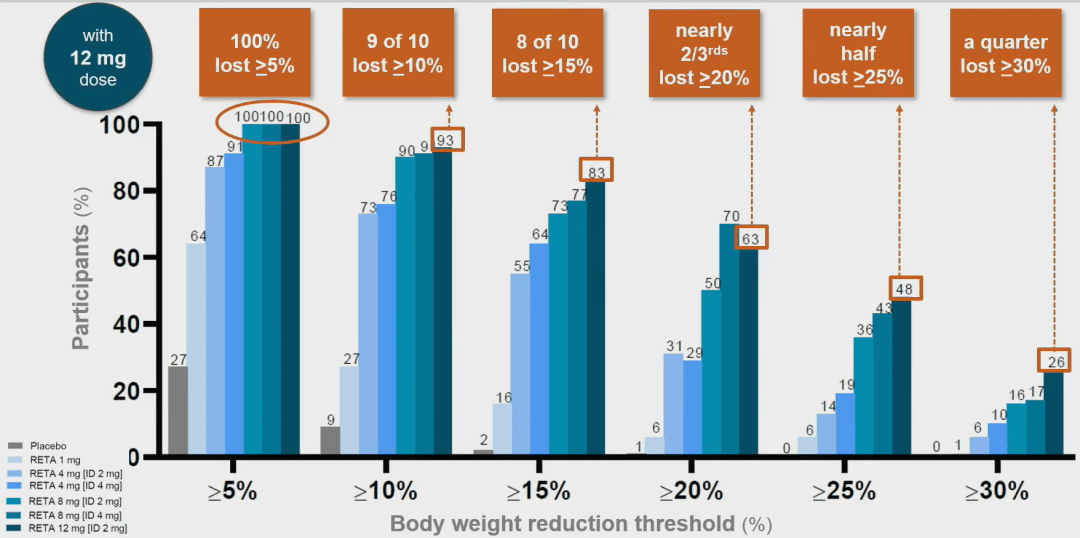
Injecting Retatrutide (12 mg) weekly, all patients can achieve weight loss of>5%; Nearly half of patients can achieve weight loss of ≥ 25%; 1/4 of patients can achieve weight loss of ≥ 30%.
When the weekly injection dose is 8 mg, all patients achieve weight loss of>5% after 48 weeks regardless of the dose.
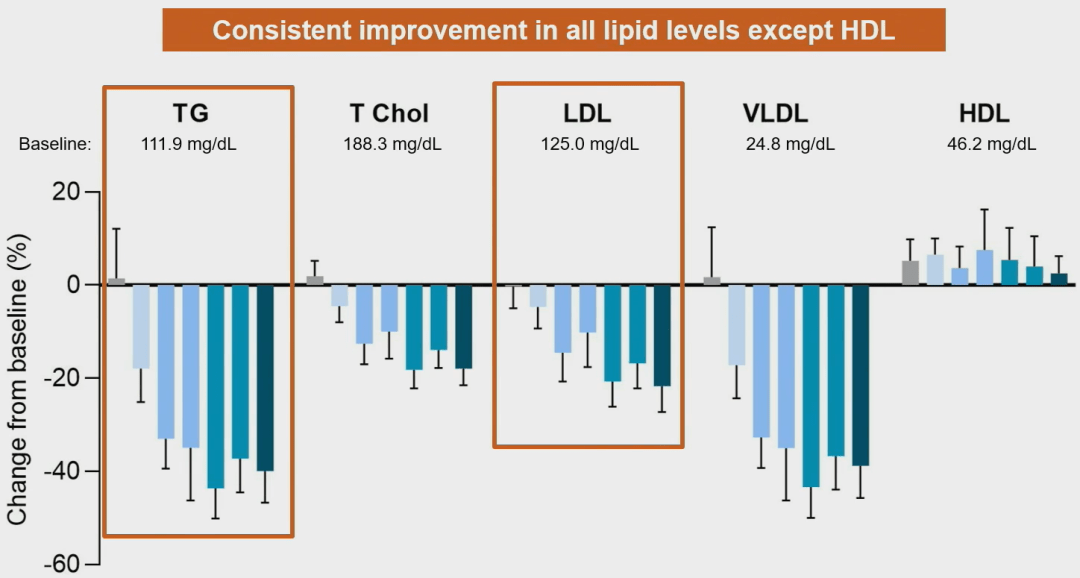
It treatment sustainably improves all lipid levels except for HDL (a 40% reduction in TG and 22% reduction in LDL), and has significant benefits for patients’ diastolic and systolic blood pressure.
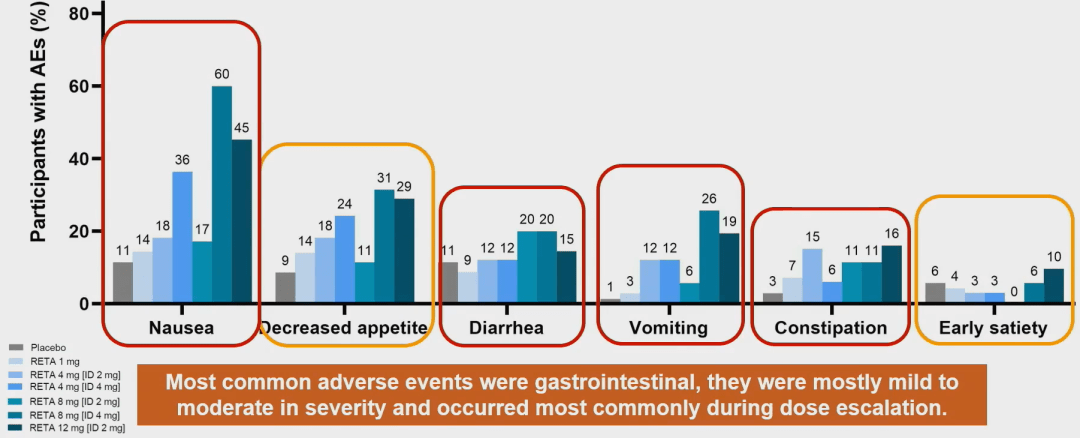
70% of patients in the placebo group reported adverse events; 73% to 94% of the Retatrutide group reported adverse events, with a higher incidence of adverse events in the 8 mg and 12 mg groups. The most common adverse event leading to discontinuation of medication is mild to moderate gastrointestinal reactions, mainly occurring during dose increases.
Retatrutide has good efficacy and tolerability in the treatment of obesity, comparable to GLP-1 and GIP/GLP-1 receptor agonists in the treatment of T2D or obesity.
Non alcoholic steatohepatitis (NASH) is a progressive form of NAFLD characterized by steatosis, inflammation, and liver cell damage, with or without fibrosis.
Currently, there are no approved drugs for treating NASH. However, research has shown that treatment based on intestinal proinsulin, including GlP-1 and GlP receptor agonists, can reduce liver fat in NAFLD patients and improve NASH related biomarkers.
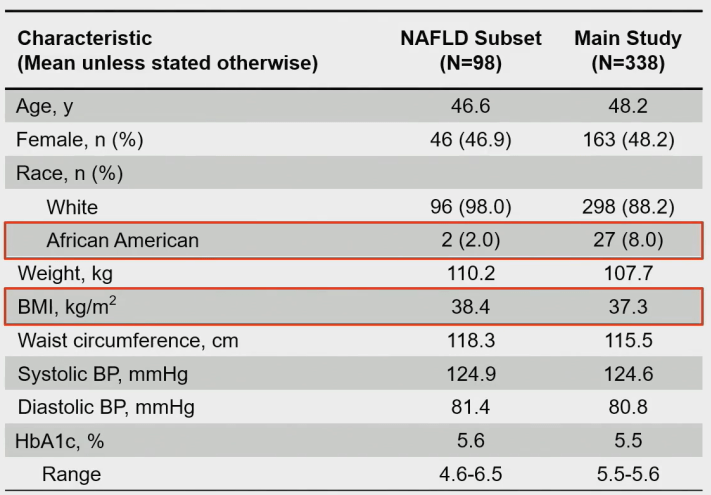
So the researchers investigated the effectiveness and safety of Retatrutide in the NAFLD subgroup. Among the 338 enrolled patients, 98 were NAFLD patients with a relatively high average BMI.
The results of the NAFLD subgroup study showed that compared to placebo, all doses of Retatrutide treatment showed a stronger liver fat reduction effect, and NASH related biomarkers (K-18 and Pro-C3) were also significantly improved.
At treatment doses of 8 mg and 12 mg, the average relative liver fat loss rate of patients was>80%;
More than 80% of patients achieve a fat loss rate of ≥ 70%; >85% of patients with liver steatosis disappear at 48 weeks.
The safety of Retatrutide in the NAFLD subgroup is similar to that in obese individuals, and no liver toxicity was found within 4 to 8 weeks of treatment.

In summary, at the highest dose of Retatrutide (12 mg), over 90% of obese and NAFLD patients achieved normalization of liver fat, indicating that adding GCG agonists to the GlP and/or GlP-1 agonists may have a stronger effect on NAFLD/NASH patients. The current pilot data and the weight loss effects of the main phase 2 trials support further evaluation of the role of Retatrutide in NASH patients.
Usually we have regular stock products, if you are interested in the product, please contact us for the latest stock list.
We have very safe special transportation line,Retatrutide Peptide products can be sold to Europe, USA,Canada, Australia and so on.
Our payment method is Bitcoin USDT and BankAccount.